The Wang Group is dedicated to developing innovative synthetic methodologies, with a strong emphasis on leveraging transition-metal catalysis to construct complex molecular architectures. Our research aims to enhance synthetic efficiency, achieve exquisite reaction selectivity, and promote sustainable chemical practices. We are particularly passionate about applying these new methods to the synthesis of biologically relevant compounds and functional materials. Our core research areas include:
1. Asymmetric Radical-Based Additions via Earth-Abundant Transition Metal Catalysis
Radicals offer a powerful avenue to rapidly introduce molecular complexity under mild reaction conditions, including photochemical and electrochemical initiation. However, achieving precise stereocontrol in radical reactions remains a significant challenge. Our group is focused on exploring radical-involved asymmetric addition reactions, offering complementary approaches to construct complex molecules and expand chemical space. We are committed to unraveling the fundamental mechanisms governing these processes by combining organometallic techniques and DFT calculations, thereby accelerating the development of robust catalytic systems and expanding their synthetic utility.
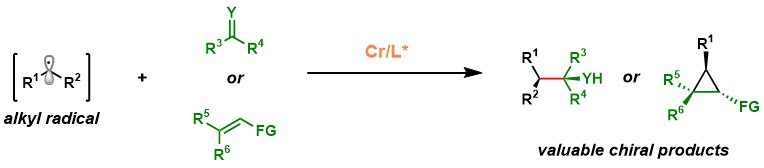
1.1 Cr-Catalyzed Radical-Based Asymmetric Carbonyl Additions:
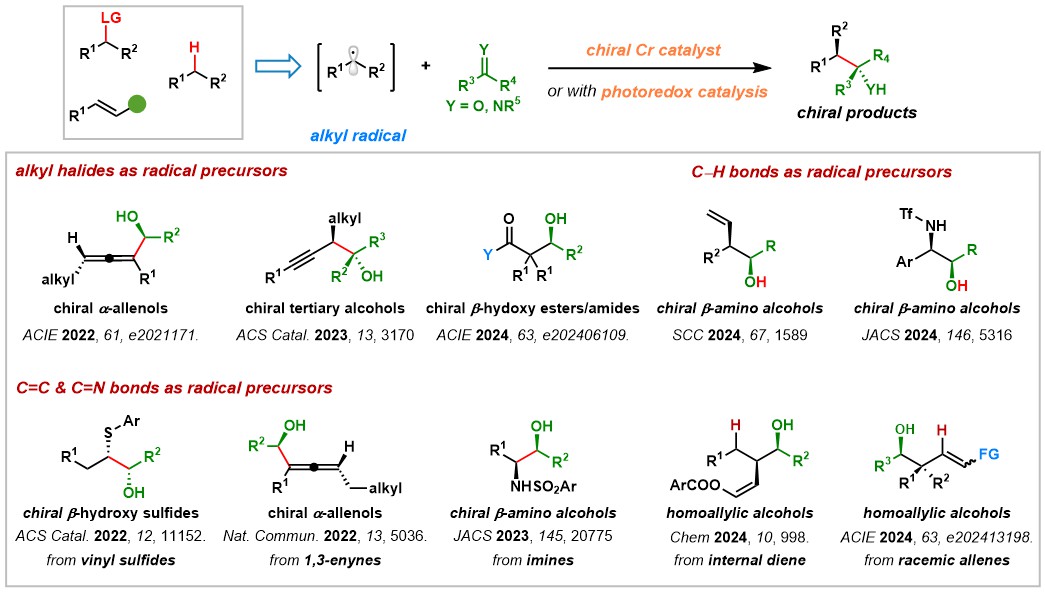
The Wang Group has advanced chromium-catalyzed radical-based asymmetric carbonyl additions, overcoming limitations in traditional Nozaki–Hiyama–Kishi (NHK) reactions. By employing readily available radical precursors, including alkyl halides, olefins, imines, and C(sp³)–H bonds, we have developed a versatile platform for constructing chiral alcohols with vicinal stereocenters. Notably, we pioneered the first Cr-catalyzed enantioconvergent reactions from racemic alkyl electrophiles and achieved asymmetric Reformatsky-type reactions through metallaphotoredox catalysis. Mechanistic investigations, utilizing techniques like radical trapping, spectroscopy, and DFT calculations, have revealed two distinct transition state models, broadening the understanding of Cr catalysis and distinguishing it from other transition-metal-catalyzed radical reactions.
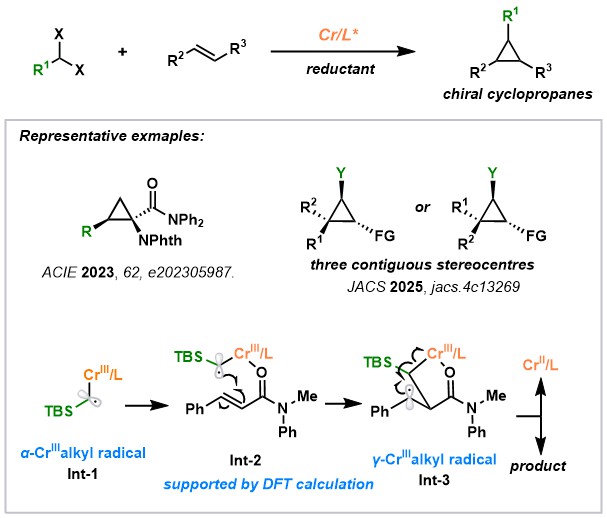
1.2 Cr-Catalyzed Radical-Based Asymmetric Cyclopropanation:
We developed the chromium-catalyzed asymmetric cyclopropanation using readily accessible gem-dihaloalkanes, circumventing the limitations of traditional diazo-based carbene transfer reactions. This method enables the synthesis of highly enantioenriched cyclopropanes, including those with three contiguous stereocenters, through a unique radical-based mechanism. Mechanistic studies, supported by DFT calculations, revealed a stepwise process involving a γ-Cr-alkyl radical intermediate, representing the first example of chromium-based metalloradical catalysis. This approach expands the scope of cyclopropane synthesis, particularly for less-stabilized carbenes, and offers promising applications in drug discovery.
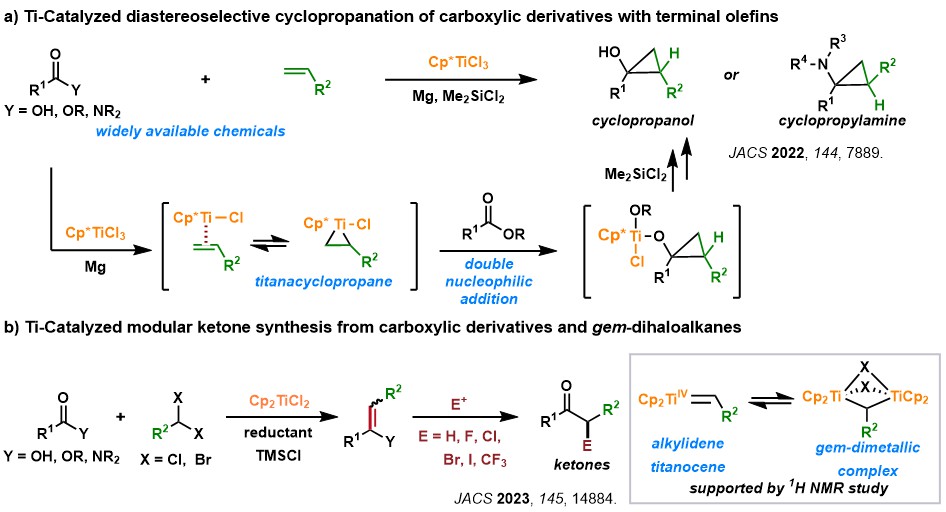
2. Titanium Catalysis: Unlocking the Potential of an Abundant and Sustainable Metal
Titanium, the second most abundant transition metal in the Earth's crust and known for its low toxicity, represents an attractive catalyst for organic synthesis. However, accessing its redox potential, particularly beyond the stable Ti(IV) state, presents a significant challenge. Our research focuses on developing novel transformations of readily available carboxylic acid derivatives, olefins, and alkynes via Ti(II)/Ti(IV) redox catalysis. We are particularly interested in exploring new reaction pathways and expanding the scope of titanium-catalyzed reactions. A deep understanding of the underlying reaction mechanisms is crucial to our efforts, enabling us to design and optimize catalytic systems for broad synthetic applications.