52. “Asymmetric Carbonyl Addition of Unactivated Homobenzyl Radicals Enabled by Non-Covalent Interactions” Shen, H.; Yu, H.; Shi, Z.; Wang, Z.*, CCS Chem. 2025, ccschem.025.202506897.
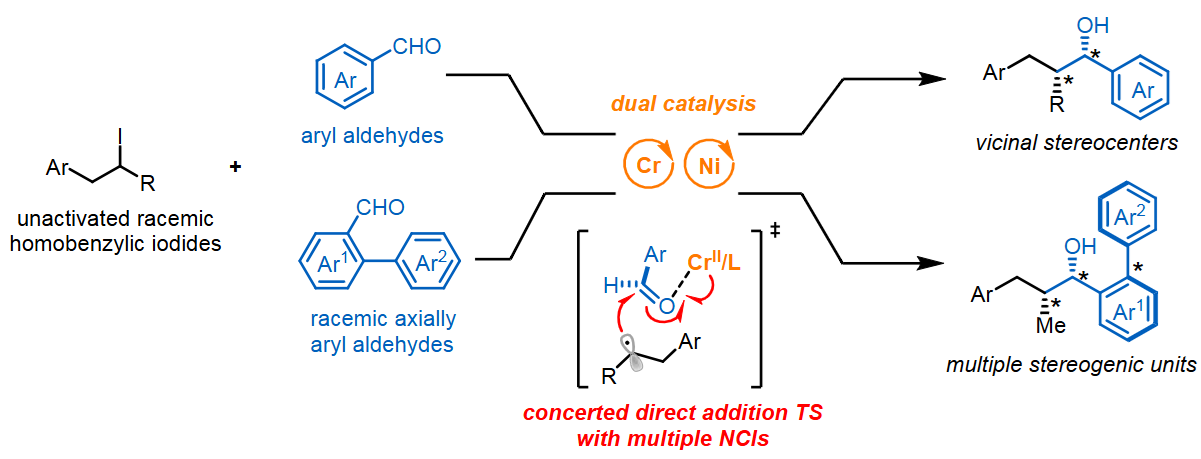
51. “Recent Advances in Cr-Catalyzed Asymmetric Nozaki–Hiyama–Kishi Reaction: From Alkyl Electrophiles to Alkyl Nucleophiles” Shen, H.; Zhang, F.-H.; Wang, Z.*, Synthesis, 2025, DOI: 10.1055/a-2726-4179.
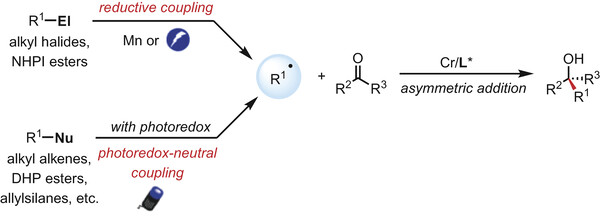
50. “Stereoselective Synthesis of Chiral Homoallylic Alcohols with Functionalized Alkenes via Radical-Involved Triple Catalysis” Lv, Y.-F.#; Shi, Z.#; Liu, G.; Shen, H.*; Wang, Z.*, ACS Catal. 2025, 15, 20157-20169. (# co-first author)
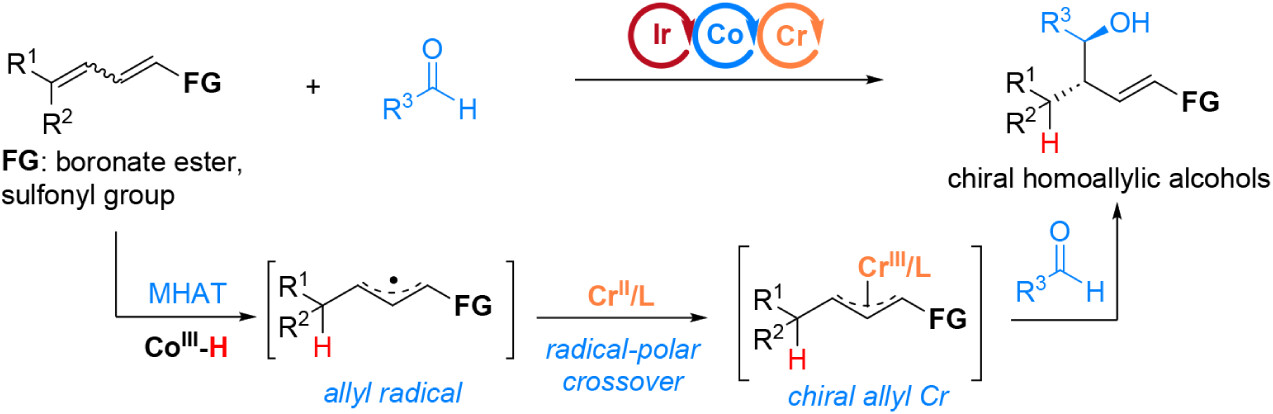
49. “Iron-Catalyzed Direct Aldehyde-to-Carbene Conversion via Ketyl Radicals” Lin, X.#; Shen, H.#*; Wang, Z.*, ACS Catal. 2025, 15, 19030-19039. (# co-first author)
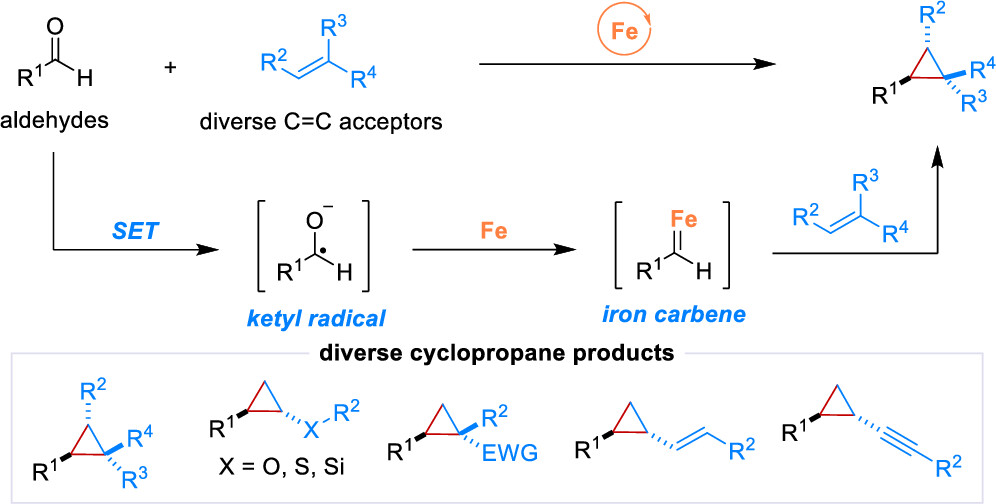
48. “Chromium-Catalyzed Radical-Involved Asymmetric Carbonyl Additions” Shen, H.; Xia, X.; Shi, Z.; Wang, Z.*, Acc. Chem. Res. 2025, 58, 2653-2670.
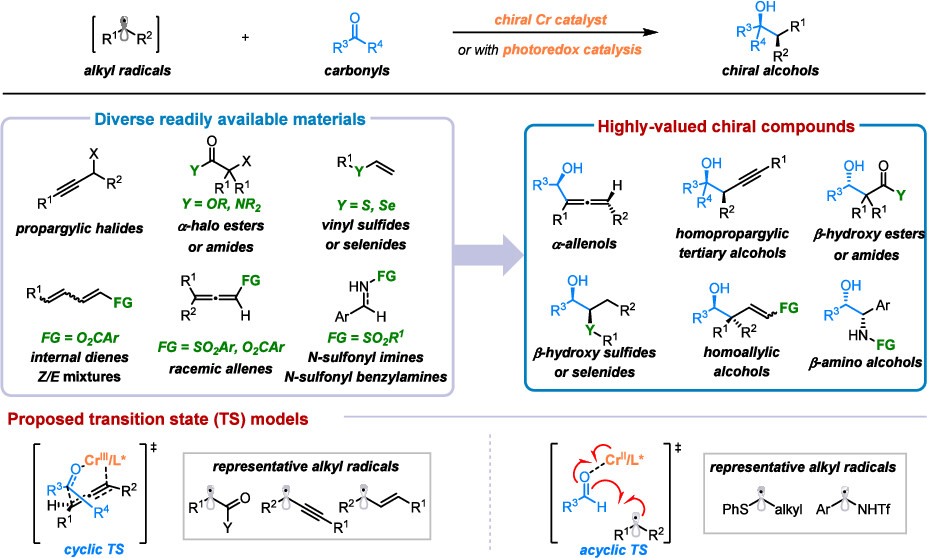
47. “Ligand-Controlled Chromium Catalysis for Tunable Z/E Selectivity in Asymmetric Homoallylic Alcohol Synthesis” Xia, X.; Yao, T.; Shi, Z.; Wang, Z.*, Angew. Chem. Int. Ed. 2025, 64, e202507474.
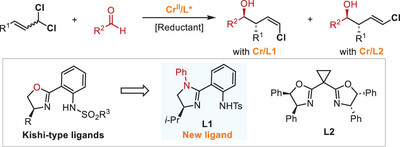
46. “Unlocking the Potential of Less-Stabilized Carbenes in Chemical Synthesis: Chromium-Catalyzed Asymmetric Cyclopropanation” Qiqige, Q.; Wang, Z.*, Synlett 2025, DOI: 10.1055/a-2655-3113. (Invited Submission)
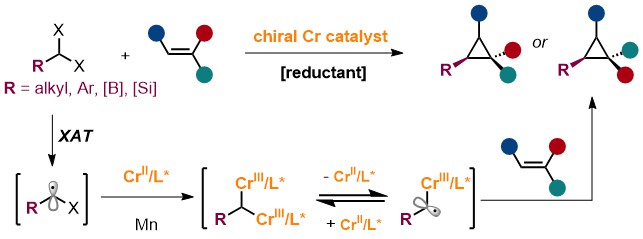
45. “Photo-Induced Stereoselective 2-Deoxyglycoside Synthesis from Glycals with Carboxylic Acids and Alcohols” Zeng, X.#; Shi, H.-Y.#; Huang, H.*; Wang, Z.*, Chin. Chem. Lett. 2025, 64, doi.org/10.1016/j.cclet.2025.111615. (# co-first author)
44. “Iron-Catalyzed Radical Allylic Substitution of Unprotected Allylic Alcohols” Liu, G.; Gao, K.; Yao, T.; Hu, H.; Wang, Z.*, Angew. Chem. Int. Ed. 2025, 64, e202500781.
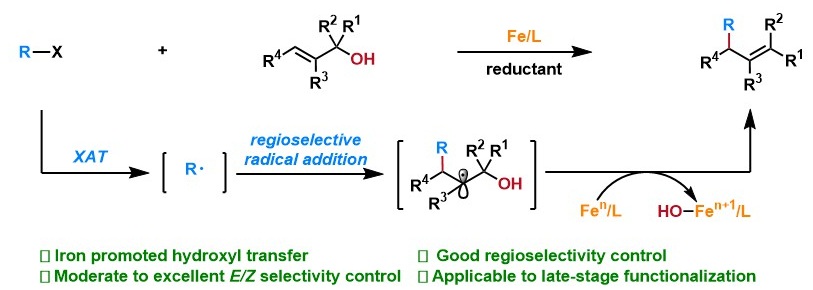
43. “A Radical Approach to Chiral β-Hydroxyboronate Esters via Synergistic Co/Cr Catalysis” Gao, K.; Wang, Z.*, ACS Catal. 2025, 15, 8454-8461.

42. “Chromium-Catalyzed Asymmetric Cyclopropanation of Aryl Alkenes with Aryl Dichloromethanes” Lin, X.; Shen, H.*; Wang, Z.*, ChemCatChem 2025, 17, e202402161. (Invited Submission)
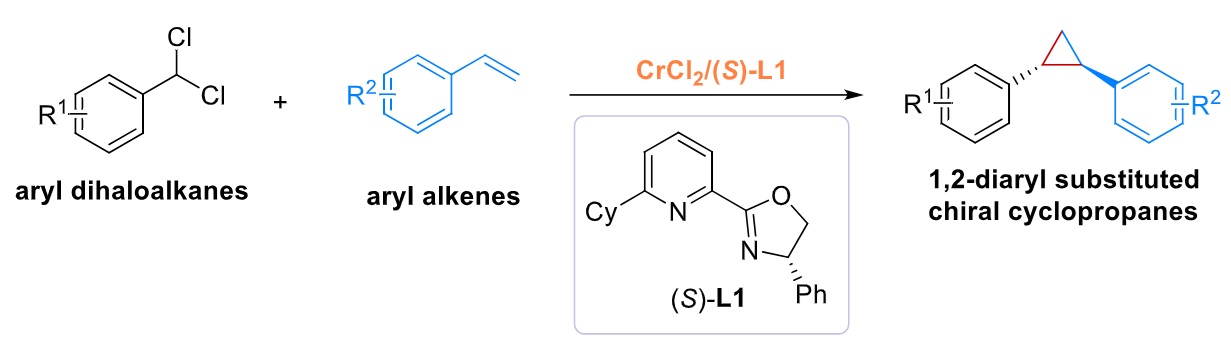
41. “Asymmetric Radical Cyclopropanation of α,β-Unsaturated Amides with α-Boryl and α-Silyl Dibromomethane via Cr(II)-Based Metalloradical Catalysis.” Wang, X.#; Shi, Z.#; Xu, M.; Lin, X.; Wang, Z.*, J. Am. Chem. Soc. 2025, 147, 7282-7292. (# co-first author)

40. "Recent Advances in Radical Hydrofunctionalization of Alkenes and Dienes via Reductive Metal-Hydride Hydrogen Atom Transfer(MHAT)" , Eur. J. Org. Chem. 2025, e202401103.
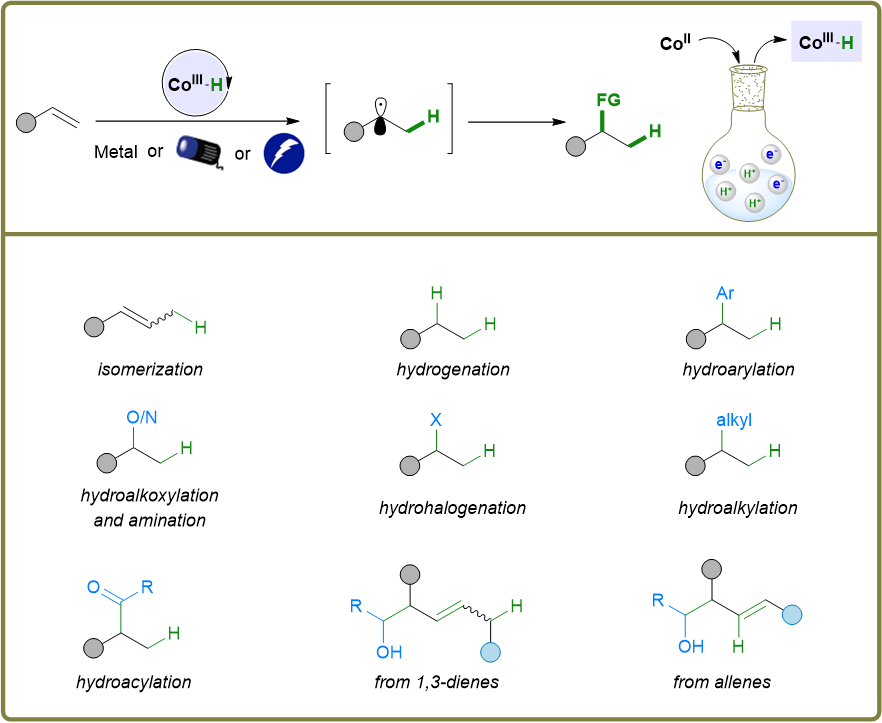
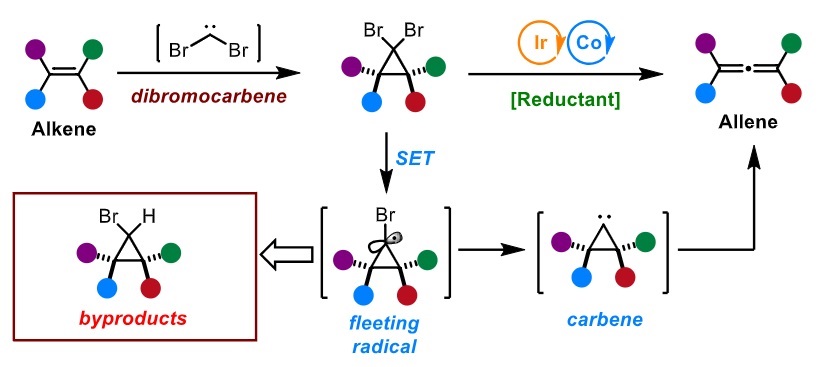
39. “Metallaphotoredox Enabled Single Carbon Atom Insertion into Alkenes for Allene Synthesis.” Liu, G.#; Shi, Z.#; Guo, C.; Gu, D.; Wang, Z.*, Angew. Chem. Int. Ed. 2025, 64, e202418746. (# co-first author)
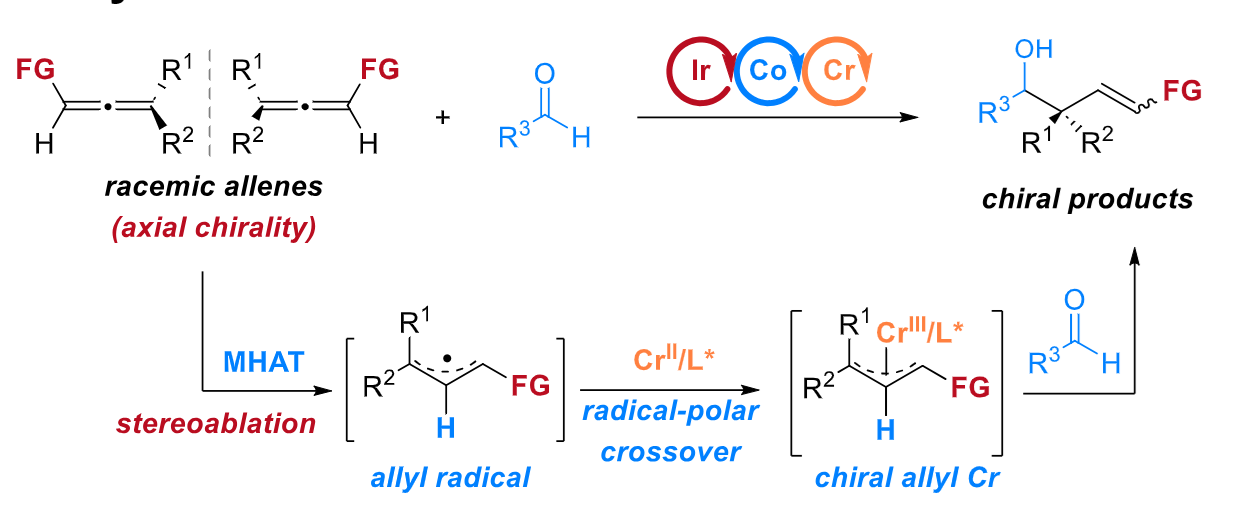
38. “Radical-Based Enantioconvergent Reductive Couplings of Racemic Allenes and Aldehydes.” Shen, H.#; Yang, L.#; Xu, M.; Shi, Z.; Gao, K.; Xia, X.; Wang, Z.*, Angew. Chem. Int. Ed. 2024, 63, e202413198. (# co-first author)
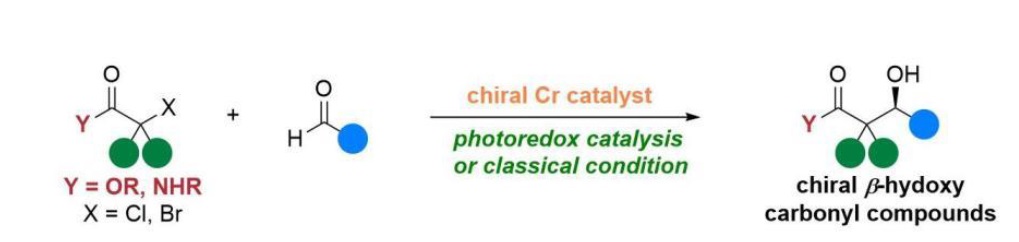
37. “Chromium Catalyzed Asymmetric Reformatsky Reaction.” Lv, Y.-F.#; Liu, G.#; Shi, Z.; Wang, Z.*, Angew. Chem. Int. Ed. 2024, 63, e202406109. (# co-first author)
36. “Fe-Catalyzed B-H and Si-H Insertion Reactions of gem-Dihaloalkanes” Wang, X.; Wang, Z.*, Org. Chem. Front. 2024, 11, 2241-2248.

35. “Access to All-Carbon Quaternary Centers by Photocatalytic Fluoroalkylation of α-Halo Carbonyl Compounds” Liu, G.; Shen, H.; Wang, Z.*, Org. Lett. 2024, 26, 1863-1867.

34. “A Radical Approach for Asymmetric α-C–H Addition of N-Sulfonyl Benzylamines to Aldehydes” Hu, H.#; Shi, Z.#; Guo, X.#; Zhang, F.-H.; Wang, Z.*, J. Am. Chem. Soc. 2024, 146, 5316-5323. (# co-first author)
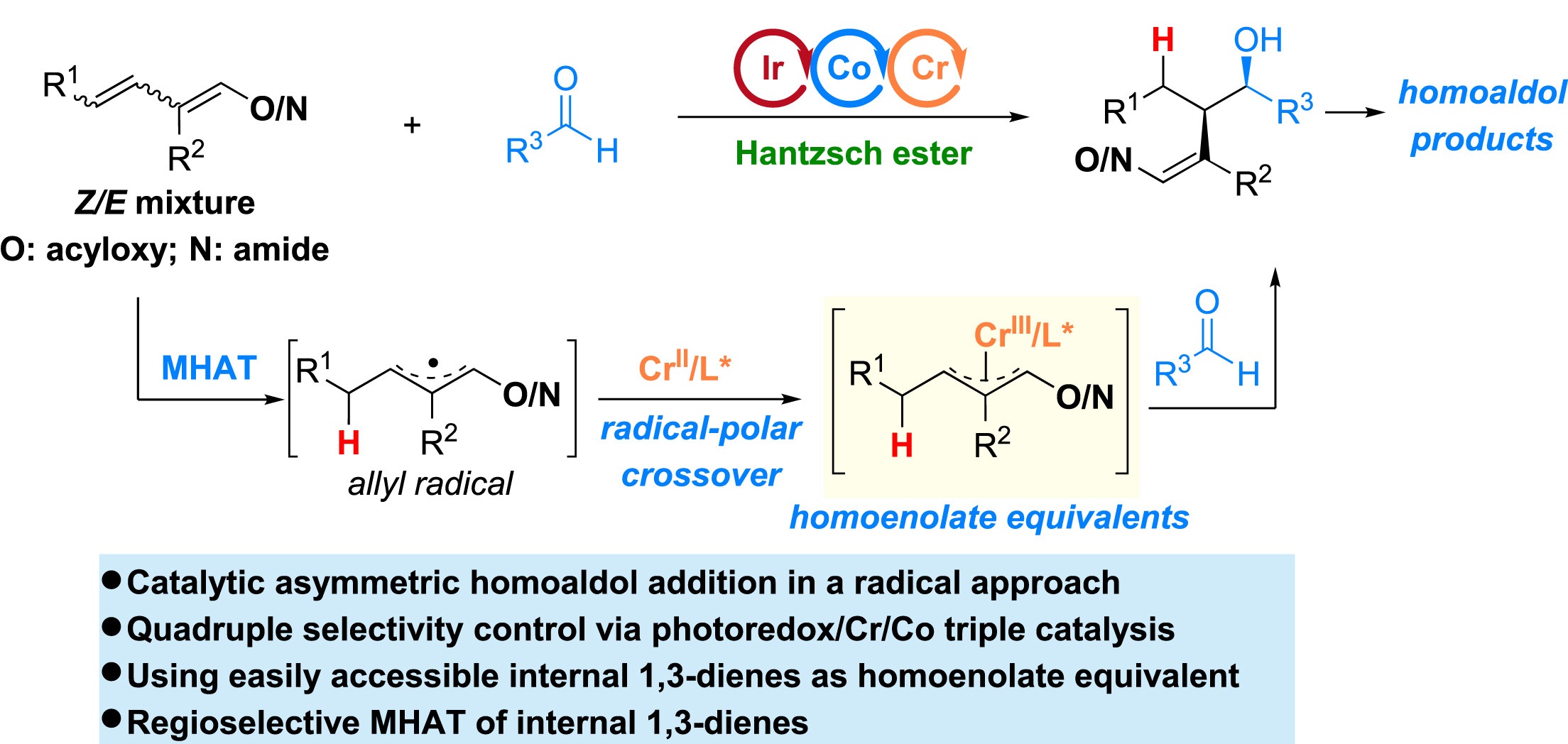
33. “A Modular Approach to Stereoselective Homoaldol Reaction via Photoredox/Cr/Co Triple Catalysis” Shen, H.; Zhang, Z.; Shi, Z.; Gao, K.; Wang, Z.*, Chem 2024, 10, 998-1014.
32. “Cr-Catalyzed Allylic C(sp3)–H Addition to Aldehydes Enabled by Photoinduced Ligand-To-Metal Charge Transfer” Zeng, X.#; Zhang, F.-H.#; Lai, R.; Lin, X.; Wang, Z.*, Sci. China Chem. 2024, 67, 1589-1595. (# co-first author)
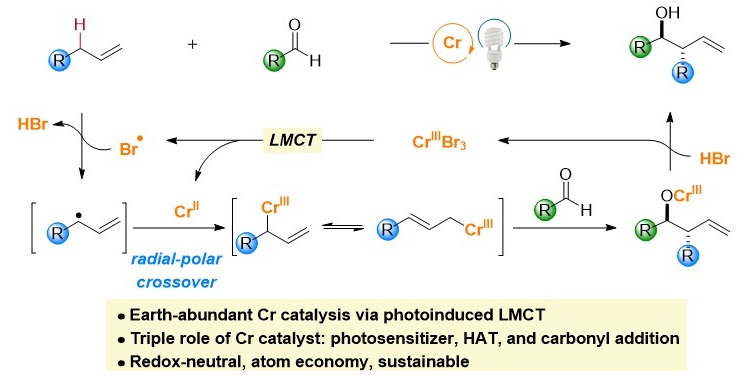
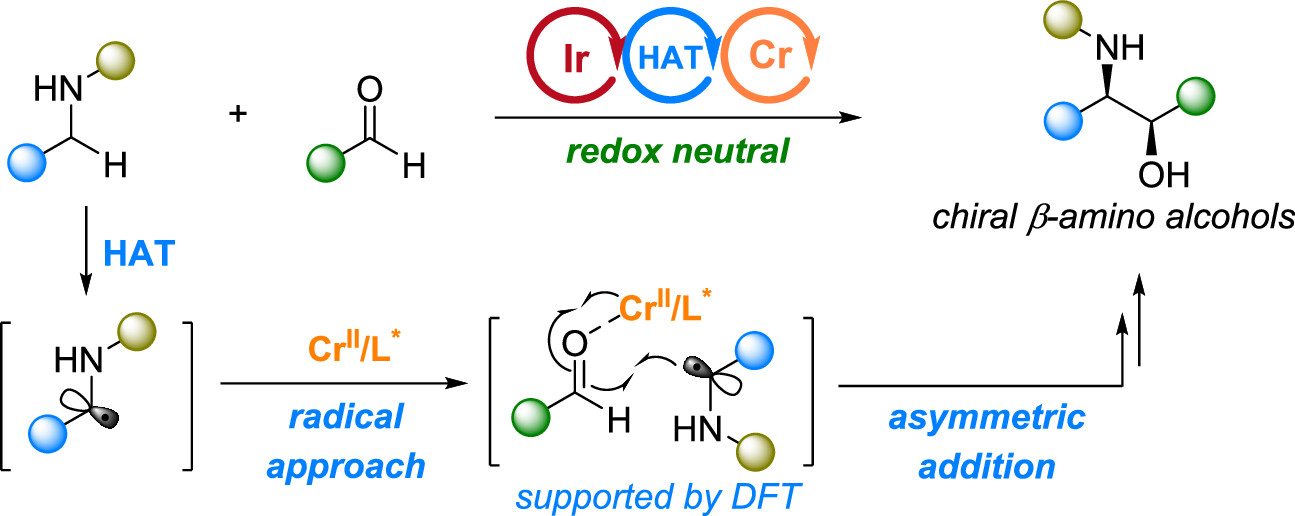
31. “Cr-Catalyzed Asymmetric Cross Aza-Pinacol Couplings for β-Amino Alcohol Synthesis” Hu, H.; Wang, Z.*, J. Am. Chem. Soc. 2023, 145, 20775-20781.
(Highlighted in Organic Process Research & Development: https://pubs.acs.org/doi/10.1021/acs.oprd.3c00460)
(Highlighted in Organic Chemistry Portal: https://www.organic-chemistry.org/abstracts/lit9/226.shtm)
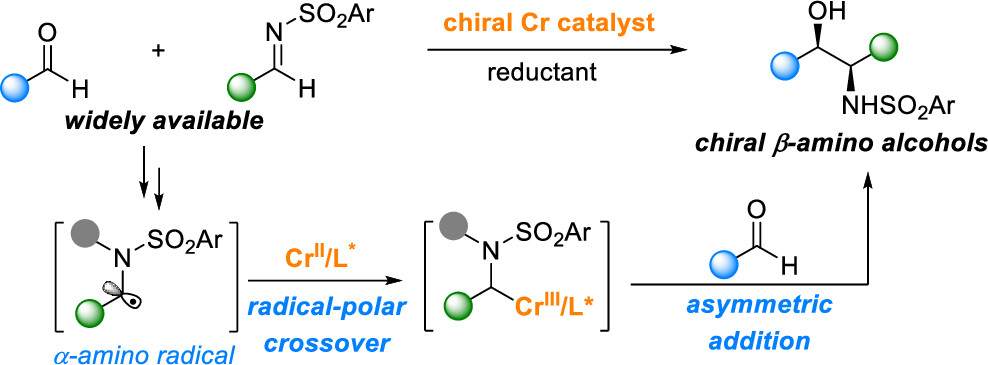
30. “Ti-Catalyzed Modular Ketone Synthesis from Carboxylic Derivatives and gem-Dihaloalkanes” Ni, J.#; Xia, X.#; Gu, D.; Wang, Z.*, J. Am. Chem. Soc. 2023, 145, 14884-14893. (# co-first author)
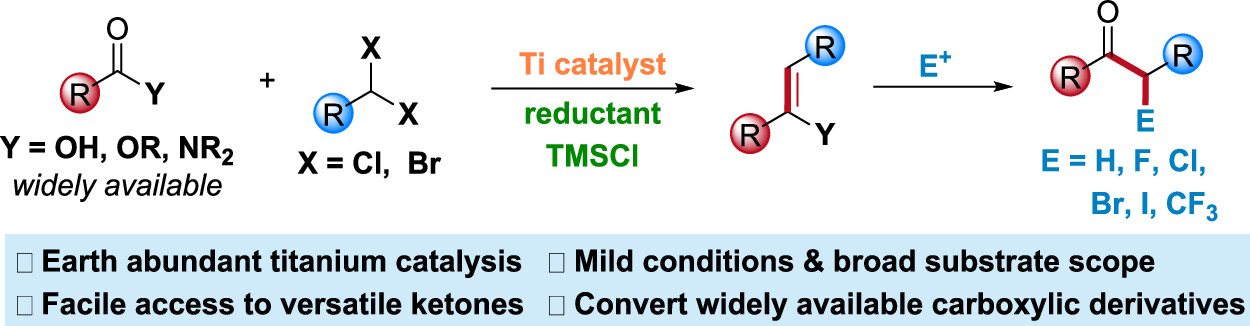
29. “Catalytic Diastereo- and Enantioselective Cyclopropanation of gem-Dihaloalkanes and Terminal Olefins” Liu, H.-L.; Wang, X.; Gao, K.; Wang, Z.*, Angew. Chem. Int. Ed. 2023, 62, e202305987.
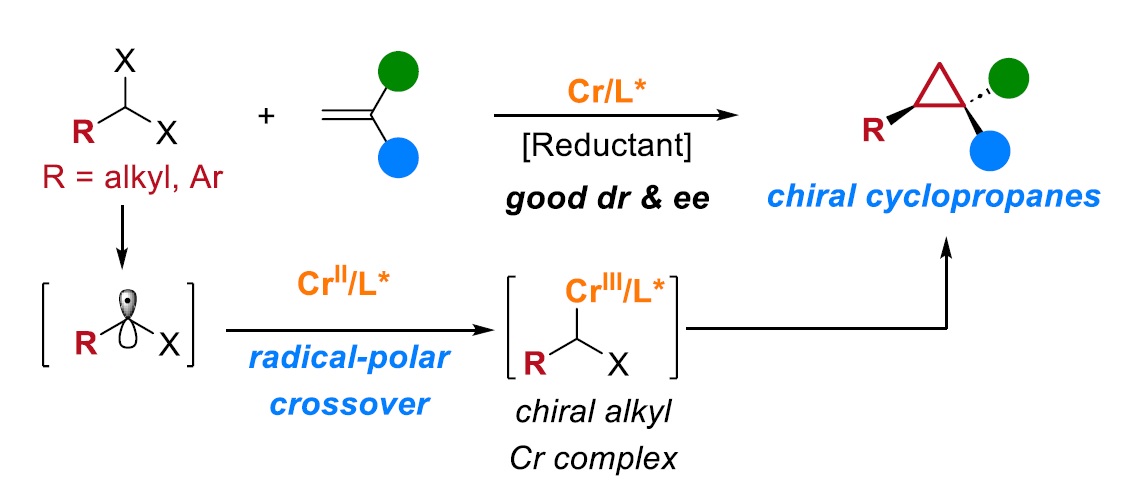
28. “Cr-Catalyzed Regio-, Diastereo-, and Enantioselective Reductive Couplings of Ketones and Propargyl Halides” Guo, X.#; Shi, Z.#; Zhang, F.-H.; Wang, Z.*, ACS Catal. 2023, 13, 3170-3178. (# co-first author)
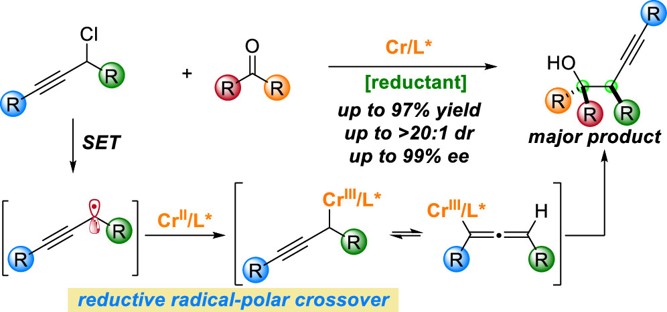
27. "Cr-Catalyzed Chiral Allenone Synthesis via Sequential Radical-Polar Crossover and Oppenauer Oxidation" Zeng, X.; Zhang, F.-H.*; Wang, Z.*, Org. Chem. Front. 2023, 10, 310-316. (Invited submission, Frontiers Emerging Investigators Series)

26. “Cr-Catalyzed Diastereo- and Enantioselective Synthesis of β-Hydroxy Sulfides and Selenides.” Xia, X.; Wang, Z.*, ACS Catal. 2022, 12, 11152-11158.

25. “Asymmetric 1,4-Funcitonalization of 1,3-Enynes via Dual Photoredox and Chromium Catalysis.” Zhang, F.-H.#; Guo, X.#; Zeng, X.; Wang, Z.*, Nat. Commun. 2022, 13, 5036. (# co-first author)

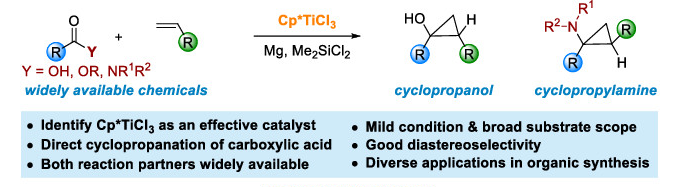
24. “Ti-Catalyzed Diastereoselective Cyclopropanation of Carboxylic Derivatives with Terminal Olefins.”Ni, J.; Xia, X.; Zheng, W.-F.; Wang, Z.*, J. Am. Chem. Soc. 2022,144, 7889–7900.
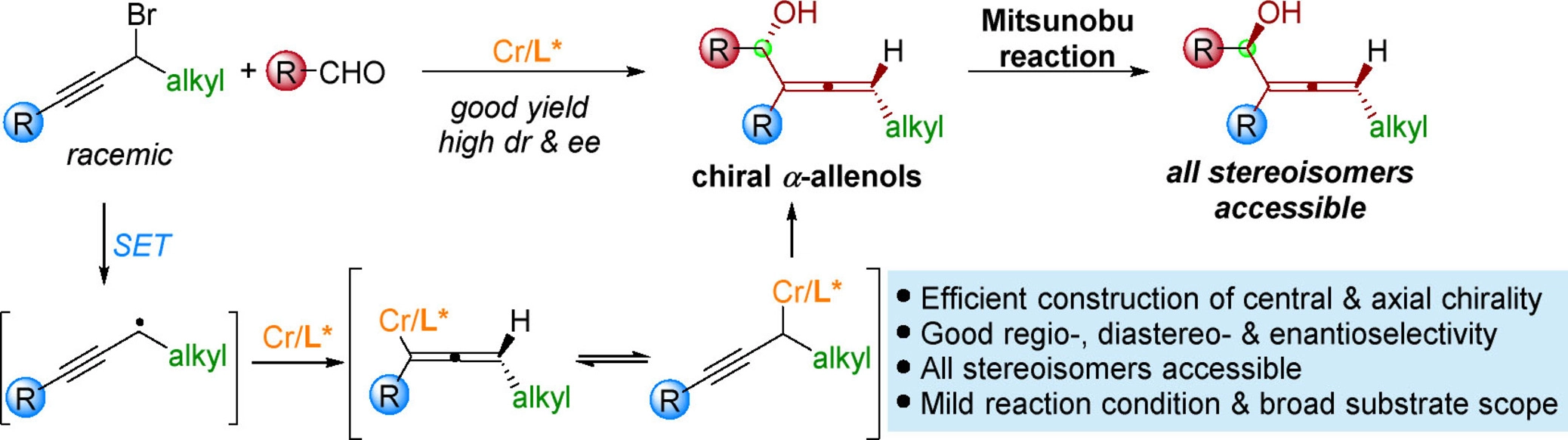
23. "Catalytic Enantioconvergent Allenylation of Aldehydes with Propargyl Halides.” Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z.*, Angew. Chem. Int. Ed. 2022, 61, e202117114.
22. “Quaternary Stereocentres via Catalytic Enantioconvergent Nucleophilic Substitution Reactions of Tertiary Alkyl Halides” Wang, Z.; Yang, Z-P.; Fu, G. C.*, Nat. Chem. 2021,13, 236-242.

21. “Catalytic Enantioconvergent Couplings of Secondary and Tertiary Electrophiles with Olefins” Wang, Z.; Yin, H.; Fu, G. C.*, Nature 2018, 563, 379−383.
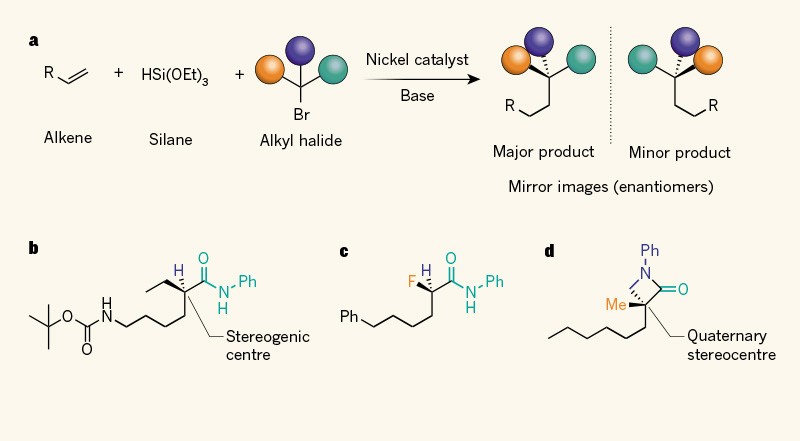
20. “Nickel-Catalyzed Enantioconvergent Borylation of Racemic Secondary Benzylic Electrophiles” Wang, Z.; Bachman, S.; Dudnik, A. S.; Fu, G. C.*, Angew. Chem. Int. Ed. 2018, 57, 14529−14532.

19. “Catalytic Asymmetric 1,6-Conjugate Addition of para-Quinone Methides: Formation of All-Carbon Quaternary Stereocenters”, Wang, Z.; Wong, Y. F.; Sun, J.* , Angew. Chem., Int. Ed. 2015, 54, 13711−13714. (Highlighted in SYNFACTS)
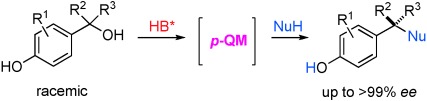
18. “Catalytic Enantioselective Intermolecular Desymmetrization of Azetidines”, Wang, Z.; Sheong, F.; Sung, H.; Williams, I.; Lin, Z.; Sun, J.*, J. Am. Chem. Soc. 2015, 137, 5895−5898.

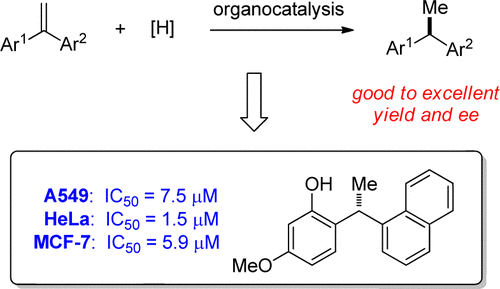
17. “Organocatalytic Asymmetric Synthesis of 1,1-Diarylethanes by Transfer Hydrogenation”, Wang, Z.; Ai, F.; Wang, Z.; Zhao, W.; Zhu, G.; Lin, Z.; Sun, J.*, J. Am. Chem. Soc. 2015, 137, 383−389.
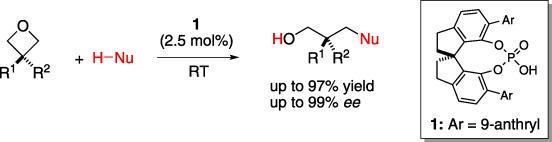
16. “Catalytic Enantioselective Intermolecular Desymmetrization of 3-Substituted Oxetanes”, Wang, Z.; Chen, Z.; Sun, J.*, Angew. Chem. Int. Ed. 2013, 52, 6685−6688. (Front cover, highlighted in SYNFACTS)
15. “Enantioselective [4+2] Cycloaddition of o-Quinone Methides and Vinyl Sulfides: Indirect Access to Generally Substituted Chiral Chromanes”, Wang, Z.; Sun, J.*, Org. Lett. 2017, 19, 2334−2337.
14. “Organocatalytic Enantioselective Desymmetrization of meso-Aziridines and Prochiral Azetidines”, Wang, Z.; Hong, W.-X.; Sun, J.*, Curr. Org. Chem. 2016, 20, 1851−1861.
13. “Recent Advances in Catalytic Asymmetric Reactions of o-Quinone Methides”, Wang, Z.; Sun, J.*, Synthesis 2015, 47, 3629−3644.
12. “Catalytic Asymmetric Nucleophilic Opening of 3-Substituted Oxetanes”, Wang, Z.; Chen, Z.; Sun, J.*, Org. Biomol. Chem. 2014, 12, 6208−6032.
11. “Chiral Phosphoric Acid Catalyzed Enantioselective Desymmetrization of meso-Epoxides by Thiols”, Wang, Z.; Law, W. K.; Sun, J.*, Org. Lett. 2013, 15, 5964−5966.
10. “An Organocatalytic Kinetic Resolution of Aziridines by Thiol Nucleophiles”, Sun, S.1; Wang, Z.1 (co-first author); Li, S.; Zhou, C.; Song, L.; Huang, H.*; Sun, J.*, Org. Lett. 2020, acs.orglett.0c04074. (1: equal contribution).
9. “Catalytic Enantioselective Synthesis of Tetrahydroisoquinolines and Their Analogues Bearing a C4 Stereocenter: Formal Synthesis of (+)-(8S, 13R)-Cyclocelabenzine”, Chen, Z.1; Wang, Z.1 (co-first author); Sun, J.*, Chem. −Eur. J. 2013, 19, 8426−8430. (1: equal contribution)
8. “Enantioselective Chloride Opening of Oxetanes: Unusual Use of Wet Molecular Sieves for Controlled Release of HCl”, Yang, W.; Wang, Z.; Sun, J.*, Angew. Chem. Int. Ed. 2016, 55, 6954-6958.
7. “Chiral Phosphoric Acid Catalyzed Asymmetric Addition of Naphthols to p-Quinone Methides”, Wong, Y. F.; Wang, Z.; Sun, J.*, Org. Biomol. Chem. 2016, 14, 5751−5754.
6. “A One-Pot Oxidation/Cycloaddition Synthesis of 2,4-Diary Chromans Via ortho-Quinone Methides”, Wong, Y. F.; Wang, Z.; Hong, W. –X.; Sun, J.*, Tetrahedron 2016, 72, 2748−2751.
5. “Organocataytic Asymmetric Nucleophilic Addition to ortho-Quinone Methides by Alcohols”, Lai, Z.; Wang, Z.; Sun, J.*, Org. Lett. 2015, 17, 6058−6061.
4. “Enantioselective Formation of All-Carbon Quaternary Stereocenters from Indoles and Tertiary Alcohols Bearing A Directing Group”, Zhao, W.; Wang, Z.; Chu, B.; Sun, J.*, Angew. Chem. Int. Ed. 2015, 54, 1910−1913.
3. “Organocatalytic Enantio- and Diastereoselective Synthesis of 1,2-Dihydronapthalenes from Isobenzopyrylium Ions”, Qian, H.; Zhao, W.; Wang, Z.; Sun, J.*, J. Am. Chem. Soc. 2015, 137, 560−563.
2. “Complex Bioactive Alkaloid-Type Polycycles through Efficient Catalytic Asymmetric Multicomponent Aza-Diels-Alder Reaction of Indoles with Oxetane as Directing Group”, Chen, Z.; Wang, B.; Wang, Z.; Zhu, G.; Sun, J.*, Angew. Chem., Int. Ed. 2013, 52, 2027−2031. (Highlighted in SYNFACTS)
1. “Synthesis of Eight-Membered Lactones: Intermolecular [6+2] Cyclization of Amphoteric Molecules with Siloxy Alkynes”, Zhao, W.; Wang, Z.; Sun, J.*, Angew. Chem., Int. Ed. 2012, 51, 6209−6213. (Highlighted by Specialty Chemicals)